What Is the Best Describes Supersaturation
Briefly describe mechanism involve during formation of precipitate. The supersaturation area is one in which the system possesses excess free energy and is not at equilibrium.

Reacting Metals And Air Freebie Middle School Science Teacher Middle School Science Classroom Teaching Middle School
The most popular example is sodium acetate which is supersaturated.
. Supersaturated solutions are extremely unstable but often require a. The solubility at 50 C is 244 g100 mL of water. Patients too can use supersaturation as a gauge of their success in managing fluids and diet.
How the Torrent of Images and Sounds Overwhelms Our Lives 2001. The warm air rises away from Earths surface and begins to cool. Needle shaped crystals will start to grow slowly.
Gitlins selection Supersaturation or The Media Torrent and Disposable Feeling comes from his book Media Unlimited. Supersaturation is achieved by dissolving a solute in one set of conditions then transferring it to other conditions without triggering any release of the solute. Physicians can and do use supersaturation to guide to treatment.
A supersaturated solution is a more solute solution than can be dissolved by the solvent. Soldier using a Hydro-Cool garment. Supersaturation occurs with a chemical solution when the concentration of a solute exceeds the concentration specified by the value equilibrium solubility.
This counter-ion layer balances the surface charge on the particle. Supersaturation in Phase Change Crystallization and Condensation Physical and chemical processes in the vapor melt or the solution phase of each system occur through the formation of three-dimensional nuclei of a new phase and take place. From even simple household experiments they can intuite its effect on crystallization.
If you havent learned what a solute solvent is the material that is dissolved in the solution such as salts but not restricted to salts is a solution. Small packs can be used either for heating or cooling depending on the material used. Supersaturation is a non-equilibrium physical state in which a solution contains more solute than the equilibrium solubility allows given conditions such as temperature and pressure of the system.
Most commonly the term is applied to a solution of a solid in a liquid. The sum of changes in enthalpy for the solute solvent and the mixture. This transition from water vapor to water droplets or ice crystals is known as condensation.
Decompression theory is the study and modelling of the transfer of the inert gas component of breathing gases from the gas in the lungs to the tissues and back during exposure to variations in ambient pressure. The colder the air gets the less water vapor it can hold. Increased temperature usually increases the solubility of solids in liquids.
B lower than the normal boiling point of water If the amount of dissolved solute in a solution at a given temperature is greater than the amount that can permanently remain in solution at that temperature the solution is said to be. For example the solubility of glucose at 25 C is 91 g100 mL of water. A heat pack contains a supersaturated solution of material such as.
A supersaturated solution is in a metastable state. -Adding more solute than a solvent can dissolve and seeing precipitate at the bottom of the solution. -Adding more solute to a solvent than would normally dissolve however upon heating everything dissolves.
A critical stage in the growth of. Supersaturation can be induced by maintaining the crystal at a lower temperature than the gas. Once it gets cold enough a process called supersaturation causes the water vapor to transfer back to a liquid or solid state.
After explaining the concepts of saturation supersaturation crystallization and seed crystals pour the supersaturated solution into a petri dish on the overhead projector stage to a depth of no more than 14. Arrow_forward Which of the following steps would affect both yield and purity during the extraction of ovalbumina. -Adding the minimum amount of solute to a solvent to cause it to be saturated.
A supersaturated solution is a solution with more dissolved solute than the solvent would normally dissolve in its current conditions. G Supersaturation describes an unstable state in which a solution contains higher solute concentration than a. In the picture above a thermal pack is applied to the back.
Molecules are more prone to leave the gas than to rejoin it so they become deposited on the surface of the container. The enthalpy change that occurs when 1 mol of gaseous solute ions is dissolved in water. Describe the process of supersaturation.
Only supersaturation can drive crystal formation and growth. 1843 19 June 2020. Add one or more seed crystals of sodium acetate trihydrate to the saturated solution.
In this selection Gitlin describes how private lives and domestic spaces have evolved from the seventeenth-century until now. The meaning of SUPERSATURATION is the state of being supersaturated. A measure of energy randomization or energy dispersal in a system.
Unable to be measured. The amount of a substance that will dissolve in a given amount of solvent. In the case of underwater diving and compressed air work this mostly involves ambient pressures greater than the local surface pressure but astronauts high altitude.
In a sense the supersaturation area resembles the transition state of a chemical reaction save that the transition state is too fleeting to detect whereas structures in the supersaturation zone may well have finite lifetimes and be detectable. Gas supersaturation is an inherently unstable state and usually leads to loss of excess gas in. This state is called supersaturation.
Gas supersaturation occurs when the total dissolved gases in a body of water exceed the concentration of total gases that can be dissolved under normal circumstances given the temperature dissolved solids and gas pressure above the water usually determined by altitude. How does a hot or a cold pack work. It may be brought to equilibrium by forcing the excess of solute to separate from the solution.
Clinical Supersaturation Use A Direct Measurement. F Mother liquor is the solution from which a precipitate is formed. A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution.
Supersaturation is also used to describe the level at which the solute concentration exceeds its solubility at given conditions expressed as. E The counter-ion layer describes a layer of solution containing sufficient excess negative ions that surrounds a charged particle. Recent Examples on the Web In these days of digital supersaturation theres a new almost yogic poignancy in dropping back into the body.

Supersaturated Solution Easy Science Chemistry Experiments Solutions Easy Science

The Change In Hydrogen Supersaturation In An Al 10wt Cu Alloy At The Download Scientific Diagram
Are Supersaturated Solutions More Conductive Than Saturated Ones Why Or Why Not Quora
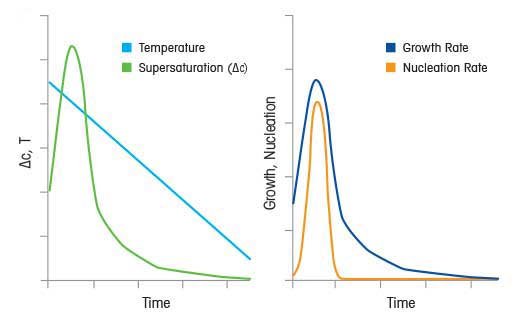
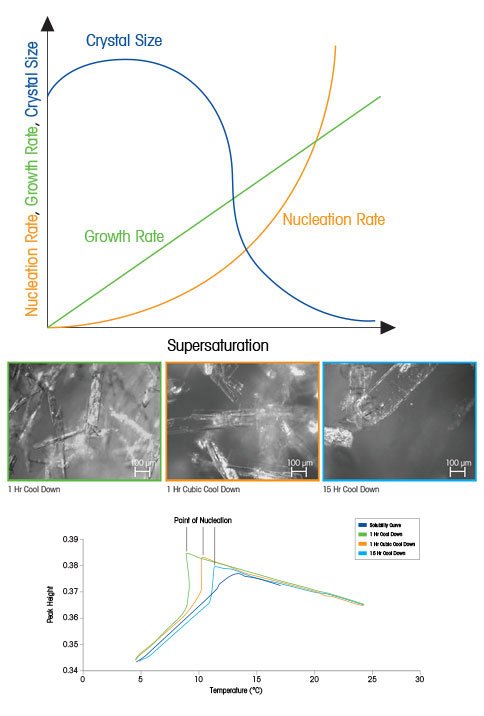
Kinetics Of Crystallization In Supersaturation

Pdf Measurement Report Cloud Processes And The Transport Of Biological Emissions Affect Southern Ocean Particle And Cloud Condensation Nuclei Concentrations
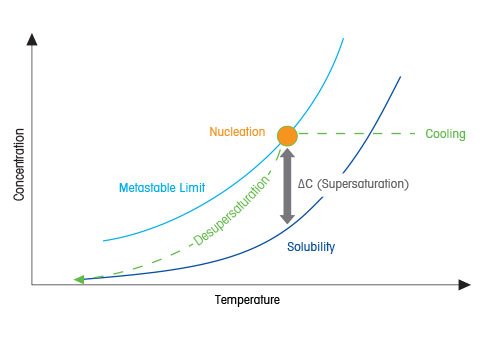
Supersaturation And Crystallization For Nucleation And Growth

Supersaturated Solutions Solutions Mechanical Energy Science Fair
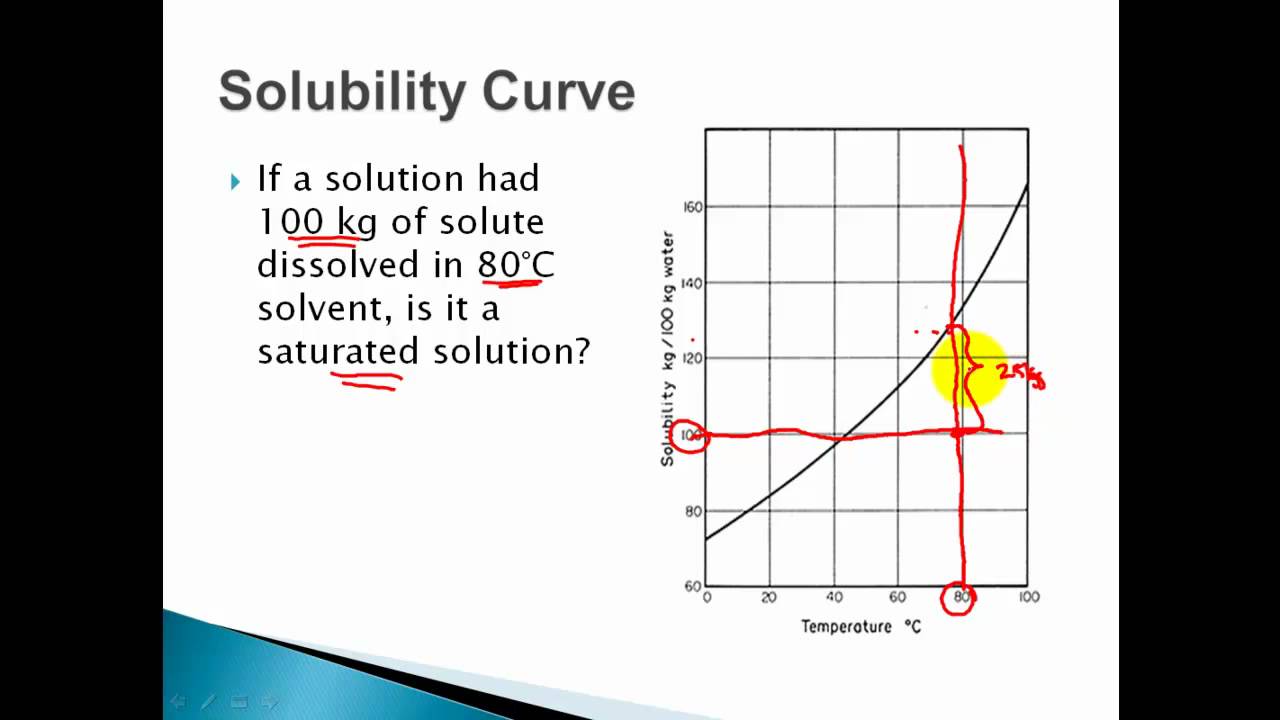
Solubility Curves Saturated Unsaturated Supersaturated Solutions Youtube
Geosciences Free Full Text Geo Heritage Specific Visibility As An Important Parameter In Geo Tourism Resource Evaluation Html

Method To Estimate Water Vapor Supersaturation In The Ambient Activation Process Using Aerosol And Droplet Measurement Data Shen 2018 Journal Of Geophysical Research Atmospheres Wiley Online Library
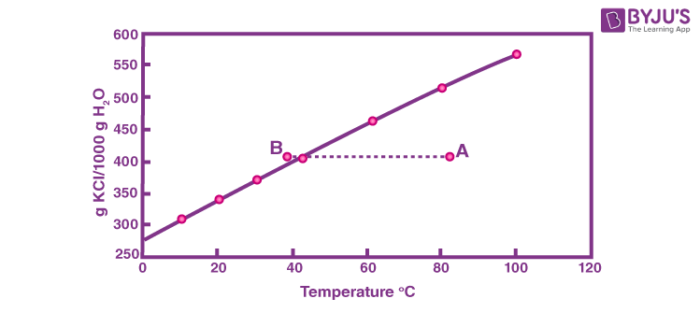
Supersaturated Solution Definition Examples Applications With Videos

Supersaturation And Crystallization For Nucleation And Growth

Supersaturated Solution An Overview Sciencedirect Topics

Supersaturation An Overview Sciencedirect Topics

Giuseppe Falini S Research Works University Of Bologna Bologna Unibo And Other Places
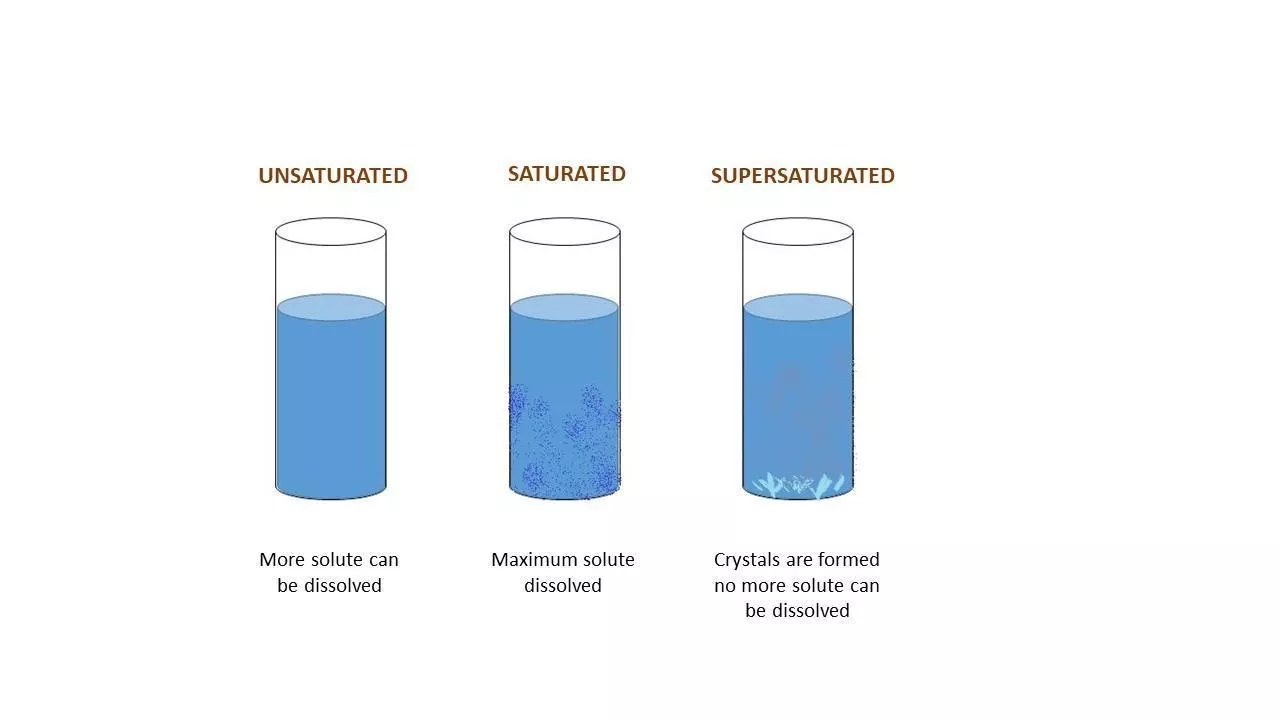
Supersaturated Solution Definition Examples Applications Faqs

Molecular Drivers Of Crystallization Kinetics For Drugs In Supersaturated Aqueous Solutions Journal Of Pharmaceutical Sciences
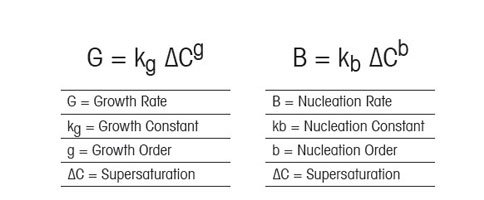
Supersaturation And Crystallization For Nucleation And Growth

Comments
Post a Comment